Survival Into the Seventh Decade of Life Following Mustard Repair
A 65-year-old woman presented to the adult congenital heart disease clinic with dyspnea on exertion and New York Heart Association functional class III limitation. She was born in 1955 with D-loop transposition (also known as dextro-transposition) of the great arteries. She underwent a Mustard operation in 1966 at age 11 years. In her 20s, she had a residual intracardiac shunt and underwent surgical baffle repair. In her 50s, she had a single-chamber pacemaker implanted for sinus node dysfunction and subsequently developed permanent atrial fibrillation. Her first heart failure hospitalization was in her 60s, and her pacemaker was upgraded to a single-chamber implantable cardioverter-defibrillator as a result of reduced systemic morphologic right ventricular (RV) systolic function. She had an additional history of recently diagnosed breast cancer with mastectomy.
A transthoracic echocardiogram showed severely reduced systolic function of both the subpulmonic morphologic left ventricle (ejection fraction 30%) and the systemic morphologic right ventricle (Figures 1A and 1B, Video 1). There were no structural valvular abnormalities, outflow obstructions, or baffle leaks. Cardiac computed tomography angiography demonstrated severe biventricular dilation, intact systemic venous baffles to the left-sided atrium, 4 intact pulmonary veins draining into the right-sided atrium by surgical conduit, a severely dilated main pulmonary artery (5.4 cm), an anomalous origin of the right coronary artery from the left main coronary artery, and a coronary artery calcium score of 165 with nonobstructive coronary artery plaques (Figures 1C to 1F).
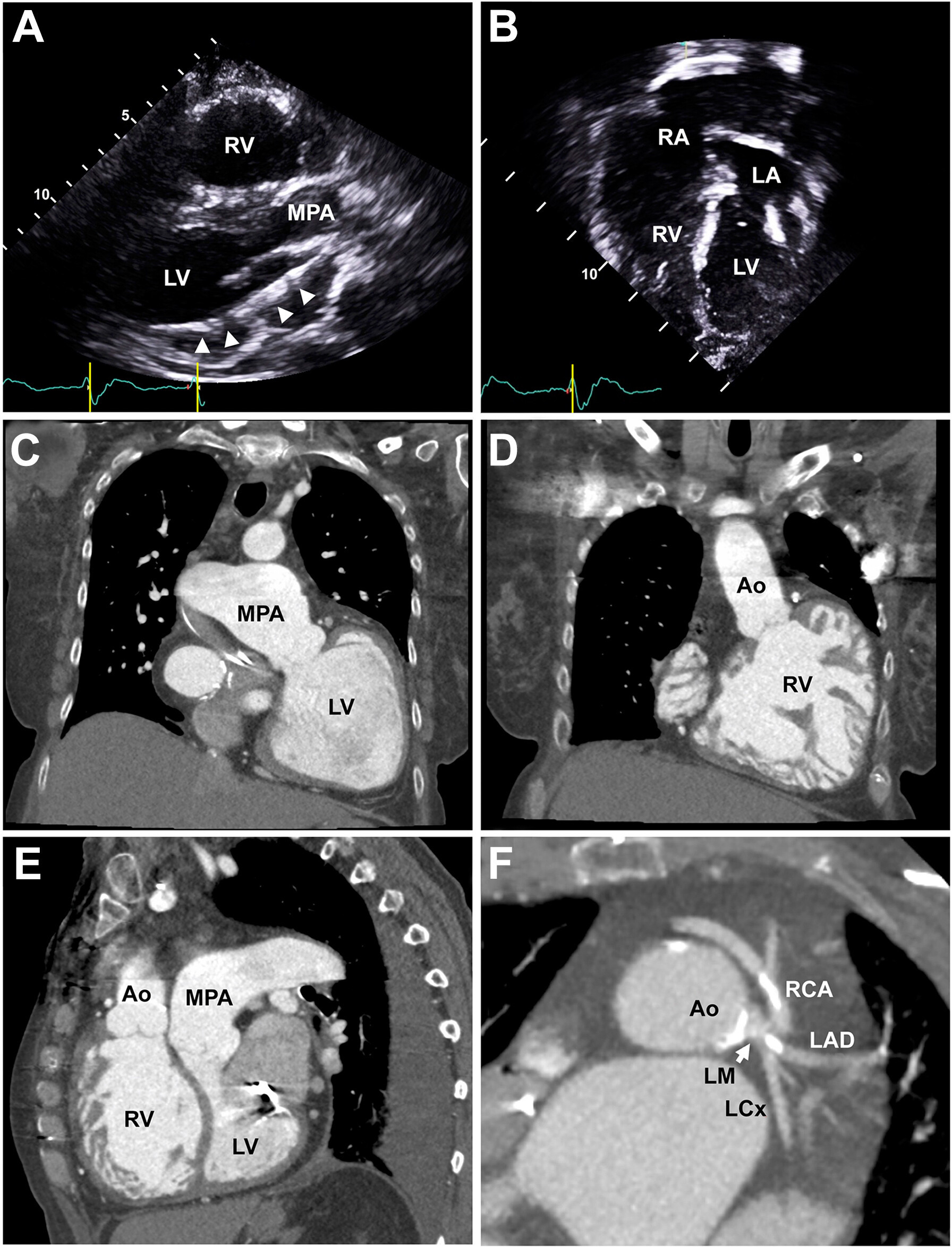
Imaging Follow-Up 54 Years After Mustard Procedure With Echocardiography and Cardiac Computed Tomography Angiography
(A) Echocardiographic parasternal long-axis view demonstrating an enlarged subpulmonic morphologic left ventricle (LV) with an implantable cardioverter-defibrillator lead (arrowheads) entering through the mitral valve into the left ventricle. (B) Apical 4-chamber view showing an intact surgical conduit directing pulmonary venous flow to the dilated systemic morphologic right ventricle (RV). (C) Cardiac computed tomography angiographic coronal view of the subpulmonic morphologic left ventricle connected to a dilated main pulmonary artery (MPA). (D) Coronal view of the dilated, hypertrophic, and hypertrabeculated systemic morphologic right ventricle connected to the ascending aorta (Ao). (E) Sagittal view demonstrating a dilated systemic morphologic right ventricle with the septum bowing into the subpulmonic morphologic left ventricle. A dilated main pulmonary artery is also noted. (F) Axial view of the aortic root and left main coronary artery (LM) (arrow) demonstrating an anomalous origin of the right coronary artery (RCA) from the left main coronary artery and calcified coronary artery plaques. LA = left atrium; LAD = left anterior descending coronary artery; LCx = left circumflex artery; RA = right atrium.
Right-sided heart catheterization showed the following: left-sided atrial pressure, 7 mm Hg; mean pulmonary artery pressure, 44 mm Hg; pulmonary capillary wedge pressure (PCWP), 21 mm Hg; pulmonary vascular resistance (PVR), 8.2 WU; cardiac output, 2.8 L/min; and cardiac index, 1.8 L/min/m2. After diuresis, her PCWP decreased to 14 mm Hg, but her PVR remained persistently elevated.
She was evaluated for heart transplantation because of severe biventricular dysfunction and worsening functional status, but she was deemed ineligible given her recent breast cancer diagnosis. She received a diagnosis of precapillary pulmonary arterial hypertension and was started on a trial of inhaled treprostinil as a last resort for treatment after implantation of a pulmonary artery pressure sensor before reconsidering transplantation options.
At the time of publication, she is 67 years of age. To our knowledge, she is among the oldest known surviving individuals to have undergone a Mustard procedure without heart transplantation. She will be reconsidered for high-risk heart transplantation after 3 to 5 years of sustained remission from breast cancer, but her biventricular heart failure and pulmonary hypertension may preclude this option. Although systemic morphologic RV failure is a common late complication of the Mustard procedure, subpulmonic morphologic left ventricular failure is unusual and may be related to her severe pulmonary hypertension. Since the widespread adoption of the arterial switch procedure, the number of surviving patients who have undergone a Mustard procedure will continue to decline. Cardiologists must be prepared to identify and manage the long-term sequelae of a systemic right ventricle.
This article is reproduced from JACC journals.
Surgerycast
Shanghai Headquarter
Address: Room 201, 2121 Hongmei South Road, Minhang District, Shanghai
Tel: 400-888-5088
Email: surgerycast@qtct.com.cn
Beijing Office
Address: room 709, No.8, Qihang international phase III, No.16, Chenguang East Road, Fangshan District, Beijing
Tel: 13331082638( Liu )
Guangzhou Office
Address: No. 15, Longrui street, longguicheng, Taihe Town, Baiyun District, Guangzhou
Tel: 13302302667 ( Ding )