The Ross Procedure: Clinical Relevance, Guidelines Recognition, and Centers of Excellence
In recent years, cumulative evidence has suggested that isolated surgical aortic valve replacement (AVR) in adults is often not curative. Whether receiving a mechanical or biological prosthesis, there follows a reduction in life expectancy compared with an age- and sex-matched population, a trend more pronounced for younger patients. In contrast, several long-term studies that examined the role of the Ross procedure in similar patient populations demonstrated restored late survival (up to 20 years) compared to a matched population. However, drawing direct conclusions from these observational studies should be tempered, because patient selection could explain some of these differences in outcomes. Nevertheless, this has spurred renewed interest into the potential role of the Ross procedure in adults.
Although the safety and excellent long-term outcomes after the Ross procedure when performed by experienced surgeons are well established, there remains a need for studies directly comparing it to alternative AVR options. The only randomized trial showed better survival, quality of life, and durability with the Ross procedure compared with aortic homograft implantation. In addition, there were a number of studies that demonstrated better survival and fewer valve-related complications with the Ross procedure compared with mechanical AVR. These differences in outcomes relate, in part, to the need for lifelong anticoagulation with a mechanical valve, thus exposing patients to a long-term risk of major bleeding, stroke, and thromboembolism, which can potentially cause devastating complications. What if no anticoagulation were required?
The study by Mazine et al in this issue of the Journal is timely and addresses long-term outcomes in patients who have undergone the Ross procedure vs bioprosthetic surgical AVR. Building on Dr David’s extensive experience with surgery in young adults, the investigators undertook a propensity-matched analysis of adults who underwent elective biological AVR vs the Ross procedure from 1990 to 2014. The patient population was relatively young, aged 18-60 years (mean: 36 years), and most patients had bicuspid aortic valve (BAV) stenosis, whereas 26% of the Ross cohort had pure aortic regurgitation. Patients with active endocarditis, acute aortic dissection, end-stage renal disease, or those who required emergency surgery were excluded. Of a total of 789 patients, 216 patients were divided into 2 matched groups, with all patients in the Ross cohort and most of those in the biological AVR cohort undergoing surgery by the senior investigator (Dr David).
At 20 years, there was a 16% difference in overall survival between the 2 groups (90% after the Ross procedure vs 74% after biological AVR; P = 0.028). Furthermore, long-term durability was significantly better after the Ross procedure. At 20 years, the cumulative incidence of any reintervention was 11.3% after a Ross procedure vs 56.8% after a biological AVR (P < 0.001). Importantly, there were no reintervention-related mortalities in the Ross cohort. In summary, long-term survival free from reintervention was significantly improved after the Ross procedure compared with biological AVR. It is of additional significance that most patients received a large bioprosthesis (78% were ≥25 mm), which would predictably lessen the risk for patient−prosthesis mismatch. These findings are remarkable in their magnitude but should again be interpreted with caution. Although propensity-matching homogenizes cohorts of patients, it can never account for unmeasured confounders, including iterative improvements in operative technique and patient selection. There were also some differences between the cohorts (although not statistically significant) that might have affected outcomes, including a more frequent history of surgery and concomitant ascending aortic replacement among the patients who underwent biological AVR. Importantly, the single-surgeon design limits the external validity of the results for nonexperts in aortic root reconstructive surgery. Last, the absence of longitudinal echocardiographic data precluded any correlations between higher mortality after biological AVR and valve performance. Nevertheless, based on these results from Canada (a country with a universal health care system), a 36-year-old patient would have a 25% risk of death 20 years after elective biological AVR, which is more than double the rate observed after the Ross procedure. Although this difference might be partially attributable to careful patient selection, as well as surgeon and institutional experience, its sheer magnitude is more likely a reflection of the fundamental differences in biology and hemodynamics between the pulmonary autograft and a prosthetic valve. This study furthers our understanding of the impact of valve choice for young adults and raises 4 points with major clinical implications.
First, although the better results of the Ross procedure compared with mechanical AVR were mostly imputed on the need for anticoagulation, the current study suggests other factors are at play. The traditional focus on differences between mechanical and biological prostheses is the proverbial tree that hides the forest. Whether biological or mechanical, all prosthetic valves lack key characteristics of living valves—they are nonliving substitutes with no ability to adapt, repair, or remodel. In addition, the rigid sewing ring immobilizes the left ventricular outflow tract and aortic annulus, thus affecting torsion of the left ventricular outflow tract and expansion of the aortic annulus, which are both critical components in cardiac dynamics, especially with exercise. In contrast, the Ross procedure provides a living autologous substitute with no prosthetic material, and the valve is a mirror image of a normal aortic valve. Autograft root physiology and flow thus mimic the normal aortic root. This study reaffirms the notion that a living valve substitute restores normal valve function, which translates into improved clinical outcomes, akin to what is observed with the repaired mitral valve.
Second, the last 2 decades have witnessed a significant increase in the proportion of biological valves implanted in young patients. Recently, this has further been fueled by the potential for valve-in-valve procedures and is often represented as a strategy for lifetime management of aortic valve disease. However, in patients younger than 60 years, this strategy warrants careful evaluation: based on multiple studies (including the current one), an initial biological AVR in this age group could be associated with lower than expected survival. In addition, current results of valve-in-valve procedures give further pause. As a result, the enthusiasm for new technologies should not distract from the primary goal of treatment at the initial operation: long-term, event-free survival with improved quality of life. In the absence of aortic valve repair or Ross expertise, a biological AVR is an adequate alternative, but patients should be informed about the potential for additional risks in the long term.
Third, there is now a large body of evidence that demonstrates a consistent signal of restored survival after the Ross procedure, and superior outcomes compared with alternative options. The study by Mazine et al adds to this body of evidence. Although these different studies only include a single randomized controlled trial, the preponderance and convergence of outcomes provide effect plausibility. In the 2020 American College of Cardiology/American Heart Association valve guidelines, there is a Class IIb recommendation for use of the Ross procedure in young adults, while there is no mention of the Ross procedure in the 2021 European guidelines. One of the reasons often cited for this is the special surgical skill and experience needed to perform the Ross operation. This raises an important question: should technical skill and availability be key considerations when recommending procedures in the guidelines? As a parallel, mitral valve repair requires specific expertise and explains the continued prevalence of mitral valve replacement in North America and Europe. Nevertheless, the guidelines accurately reflect its role in the asymptomatic patient, but only on the condition of access to expertise in mitral valve repair. As it becomes increasingly clear that prosthetic AVR in the young may result in loss of life expectancy, it is perhaps timely to revisit the role of the Ross procedure in appropriately selected patients.
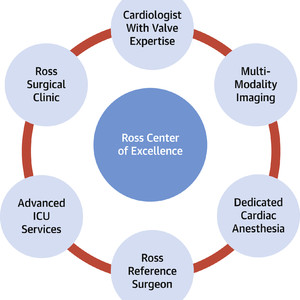
Last, much like mitral valve repair 2 decades ago, access to the Ross procedure remains limited. In addition to important geographic and socioeconomic barriers for patients, the Ross procedure is without a doubt a more complex operation than conventional AVR. Although operative risk is higher when performed in low-volume centers, there is no additional risk associated with the operation in a high-volume setting. Thus, this begs the question: has the time come for Ross Centers of Excellence (Figure 1)? This appears reasonable because of the need for advanced imaging, patient selection, and surgical expertise. Defining the latter is critical for patients to know, that if they opt for a Ross procedure, they are not compromising their own safety or the efficacy of the operation. Defining a Ross center of excellence should be based on an approved set of criteria, including case volumes, operative mortality, echocardiographic outcomes, and longitudinal follow-up, all information that should be accessible to the public. Although valve centers of excellence will have most of the components required, the main limitation will be surgical expertise and experience. This must be addressed responsibly through training and peer-to-peer support by expert Ross surgeons. Cardiologists will play an important role in expanding the accessibility to the Ross procedure, by appropriate referral to selected surgeons in their center or region so Ross experience can be concentrated, while patient safety remains the foremost concern. In this era of large data collection, patients deserve no less than to know the scientific community is transparent, and importantly, dedicated to their safety, health, and wellness.

Key Components of a Ross Center of Excellence
This article is reproduced from JACC journals.
surgerycast
Shanghai Headquarter
Address: Room 201, 2121 Hongmei South Road, Minhang District, Shanghai
Tel: 400-888-5088
Email:surgerycast@qtct.com.cn
Beijing Office
Address: room 709, No.8, Qihang international phase III, No.16, Chenguang East Road, Fangshan District, Beijing
contact number:13331082638(Liu Jie)