Adherence to Evidence-Based Therapies in Heart Failure: Deepening the Implementation Divide
Recent advances in pharmacotherapy for heart failure with reduced ejection fraction (HFrEF) have now afforded multiple, highly efficacious, life-prolonging treatments for this high-risk population. Included in these evidence-based therapies is a novel angiotensin receptor neprilysin inhibitor, sacubitril/valsartan. The PARADIGM-HF (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) clinical trial demonstrated that sacubitril/valsartan, as compared with enalapril, reduced the risk of cardiovascular death or heart failure hospitalization by 20% among ambulatory HFrEF patients. Subsequent data have determined this therapy to improve quality of life, to be safe during in-hospital initiation, and to be highly cost-effective. Regrettably, many eligible patients never receive this therapy or do so at significant delay. These implementation gaps have been well described for sacubitril/valsartan and other HFrEF therapies. However, initial prescription of these therapies represents just one aspect of the overall implementation gap—to ultimately exercise their intended population-level benefit, prescribed therapies must also be regularly taken by patients. Therefore, determining early treatment adherence rates after initial prescription represents an important metric in identifying and mitigating gaps in evidence-based care delivery.
In this issue of JACC: Heart Failure, Carnicelli et al expand our understanding of adherence patterns to sacubitril/valsartan in patients with heart failure. Using data from the American Heart Association’s Get With the Guidelines–Heart Failure registry linked with Medicare claims, the authors estimate sacubitril/valsartan adherence using a commonly used metric, the proportion of days covered (PDC), over 90 days after initial prescription. The study finds that a small minority of patients had high adherence (defined as PDC >80%) to sacubitril/valsartan, with >25% never filling the drug. The study also assessed the association between sacubitril/valsartan adherence and clinical outcomes. Poor adherence was associated with a greater risk of 1-year clinical outcomes including all-cause mortality (for PDC >80% vs ≤80%, HR: 0.42; 95% CI: 0.22-0.79). The authors appropriately conduct a sensitivity analysis excluding patients with a PDC = 0%, which may include patients who were unable to access sacubitril/valsartan caused by prior authorization rejections, excess cost, or other logistical factors. The findings remained largely consistent with some attenuation of the association after these exclusions.
Low adherence to sacubitril/valsartan, while alarming, is not entirely surprising. Similar trends in early treatment discontinuation have been consistently observed across cardiometabolic medicine. Reasons for suboptimal adherence are likely complex, and consist of patient-, provider-, and systems-level factors that promote early discontinuation or inconsistent use. Treatment-related hypotension or other hemodynamic limitations, the increased burden twice daily dosing, and high out-of-pocket costs may all further contribute to the adherence patterns observed for sacubitril/valsartan in the study. These issues deepen the growing divide between evidence generation and widespread and consistent population-level implementation in HF.
The authors observed a large association with reduced mortality rates in those with high versus low adherence to sacubitril/valsartan. While the beneficial effects of this therapy (and others) in HFrEF have been well established, the potential for significant unmeasured confounding remains to partially explain the magnitude of observed benefit. Despite robust multivariable adjustment, important differences in clinical and socioeconomic factors may still exist between those with high versus low adherence to sacubitril/valsartan. These may include greater observance of additional health-promoting behaviors, including differential diet, physical activity, and access to care considerations among patients with higher treatment adherence. Importantly, patients with high adherence to sacubitril/valsartan may also be more adherent to other HFrEF therapies. Therefore, the observed effect estimate on clinical outcomes may in this study actually be more reflective of global HFrEF therapeutic adherence rather than the effects of sacubitril/valsartan adherence alone. Additional adjustment for adherence rates to other guideline-directed medical therapy (GDMT) for HFrEF may help to further isolate the association of adherence to sacubitril/valsartan on clinical outcomes.
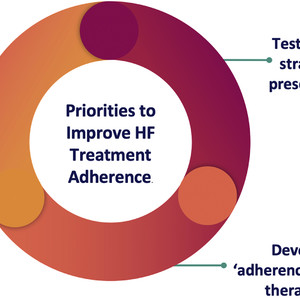
Despite the well-acknowledged limitations of observational analyses, the paper addresses a critically important topic. To derive the full potential population-level benefit that GDMT for HFrEF might offer, implementation gaps must be identified and closed not only in the prescription of evidence-based therapies, but also in their sustained use. Recent evaluations have assessed several strategies to augment HFrEF GDMT prescription. These interventions have often been rooted in behavioral economic concepts, and have included patient-activation via pre-visit checklists, , inpatient “GDMT teams” offering virtual therapeutic optimization suggestions to those caring for heart failure patients, and audit-and-feedback approaches. While continued investments in studying the impact of these ’nudges’ are urgently needed, the findings of this study suggest that strategies focused solely on initial prescription may be insufficient, as prescribed therapies are often intermittently taken or discontinued early. What more needs to be done to promote sustained treatment adherence after initial prescription?
First, higher-fidelity approaches to measure adherence are needed. Current metrics, including PDC and others, capture fill patterns as a surrogate for medication use. Complexities including the use of multiple pharmacies, duplicate prescriptions, and stockpiling may therefore misclassify adherence rates. In addition, many patients may fill prescriptions regularly but take medications only intermittently, leading to an artificially inflated PDC. Better measurement strategies may leverage advances in technology, including electronic monitoring capable of determining when pill bottles are opened or pillboxes are accessed. As these approaches will initially be expensive and challenging to scale, identification of patients at highest risk for treatment discontinuation and adverse events will be important for prioritization. However, sustained technology investments in health will likely soon improve existing technologies and democratize these approaches at more reasonable costs. In addition, dashboards (for patients and providers) that provide adherence information grouped by disease condition (eg, heart failure, coronary artery disease) might help. Grouping may reinforce and educate patients on treatment indications and help readily identify barriers to the use of particular therapies ahead of specialty ambulatory encounters.

A Framework for Improving Adherence to HF Therapies
Second, coupled with more accurate measures of medication adherence, implementation trials should be prioritized. Nudges to improve adherence—aimed to guide, motivate, and remind patients—should be empirically evaluated for efficacy. Software in exercise bicycles has the capability to remind riders when they have missed consecutive days of exercise—why not trial similar approaches in health care to detect early gaps in adherence, and nudge patients (and/or providers) accordingly? In addition, rewards or gamification elements have augmented participation in other healthy behaviors such as physical activity, even among older adults. Peer networks might also encourage sustained adherence to therapy. These models will ultimately require calibration to limit alert fatigue, but represent small investments which could have outsized impact on patients. Ideally, proposed interventions should be rooted in behavioral psychology theory that is aimed at developing, sustaining, and reinforcing positive habits around medication adherence.
Finally, we must acknowledge that the pace of scientific innovation in HFrEF has the unintended consequence of increasing the pill burden for patients. This issue is further compounded by a growing comorbid burden in heart failure, including many conditions that have their own sets of evidence-based therapies. The result is that polypharmacy in the current landscape is largely inevitable for the well-treated HFrEF patient; however appropriate, polypharmacy may ultimately reduce treatment adherence and curtail the benefit that these therapies would otherwise offer. Novel therapeutic delivery platforms could offer important solutions. Fixed-dose combination therapies have been developed for lipid lowering; calls for similar combinations (integrating sacubitril/valsartan and other therapies) have been made in heart failure. Likewise, therapeutics with longer durations of action such as those for lipid reduction, could extend dosing intervals to weeks or months. Gene editing platforms for cardiovascular disease are also under development, offering the potential for durable benefit from a single administration. These “adherence-informed” therapeutics represent an important new frontier in heart failure and highlight the recognition that therapeutic benefit is a function of the overall continuity of treatment exposure.
Patients with HFrEF may now benefit from several treatments that incrementally reduce morbidity and mortality. For these innovations to exert their greatest population-level benefit, we must extend our thinking of implementation science to prioritize both the prescription and long-term adherence of these therapies. The effort should be well worth it.
This article is reproduced from JACC journals.
surgerycast
Shanghai Headquarter
Address: Room 201, 2121 Hongmei South Road, Minhang District, Shanghai
Tel: 400-888-5088
Email:surgerycast@qtct.com.cn
Beijing Office
Address: room 709, No.8, Qihang international phase III, No.16, Chenguang East Road, Fangshan District, Beijing
contact number:010-5123-5010 13331082638(Liu Jie)