Encountering another aneurysm after aortic dissection surgery!
Two years ago, the patient suffered a fatal aortic dissection and narrowly survived through open chest surgery; Two years later, the residual abdominal aortic dissection continued to dilate and form an abdominal aneurysm, severely restricting blood supply to important organs and once again hanging on the brink of death. Professor Deng Hongping, Director of the Department of Vascular Surgery at Wuhan University People’s Hospital (Hubei Provincial People’s Hospital), and his team innovatively used the "Five Windows and One Thread" technology to successfully remove the "irregular time bomb" in the abdominal cavity of Mr. Liu, a patient from Shiyan, Hubei Province, and once again pulled him back from the "gate of hell".
Dangerous:
Two years after aortic dissection surgery, recurrent abdominal aneurysm
Mr. Liu, 55 years old, underwent a sudden aortic dissection two years ago and underwent a "cardiac arrest+extracorporeal circulation" thoracic aortic artificial vessel replacement surgery at a local hospital. His life was saved, but he developed cerebral hemorrhage and renal failure after surgery. After multiple treatments, he improved.
The significant impact of open chest surgery and the dangerous experience of multiple hospitalizations have caused Mr. Liu enormous physical and psychological trauma. In addition, the results of the follow-up examination indicate that the residual aortic dissection is gradually expanding, forcing him to retire early and recuperate at home.
During the postoperative follow-up examination in May this year, it was found that Mr. Liu’s abdominal aortic dissection had significantly expanded into an aneurysm, and blood supply to internal organs such as the kidneys was restricted. Mr. Liu and his family, who were concerned about the high risk of undergoing another surgery, sought medical attention from Director Deng Hongping of the Department of Vascular Surgery at Wuhan University People’s Hospital.
Based on the results of the aortic CTA examination, Professor Deng Hongping found that Mr. Liu had residual aortic dissection dilation at the distal end of the surgical site, forming aneurysms from the origin of the abdominal aorta to both iliac arteries, with a high risk of rupture and massive bleeding.
Although traditional surgery is effective, it involves significant trauma, high risk, heavy bleeding, and slow recovery. On the other hand, minimally invasive endovascular surgery is extremely difficult to isolate the aneurysm and prevent visceral artery ischemia. Even a slight mistake during surgery may lead to serious consequences such as kidney loss and intestinal ischemic necrosis.
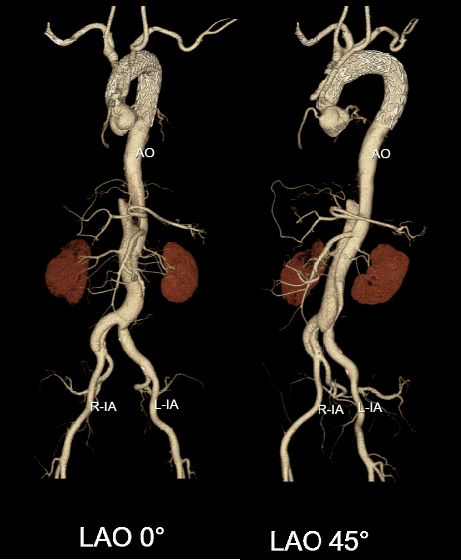
Preoperative CTA
Breach:
Innovative cutting-edge techniques, ’Five Windows and One Thread’ to save lives
The general discussion of vascular surgery organization believes that the patient has a huge abdominal aortic dissection aneurysm, and the arterial interface responsible for supplying blood to important organs is entirely located inside the aneurysm cavity. Therefore, only the "four window" surgery, which is the "ceiling level" surgery in the field of aortic disease in the world, can be performed.
In addition, Mr. Liu’s right renal artery is supplied by a false lumen, and during the surgery, a guide wire needs to pass through the aortic true lumen, pass through a dissection, and enter the false lumen, and then selectively enter the right renal artery true lumen, which greatly increases the difficulty of the surgery. Unlike ordinary people, Mr. Liu has two left renal arteries, which means that the surgery requires the reconstruction of all five visceral blood vessels, further increasing the difficulty and risk.
The difficulty in treating abdominal aortic dissection aneurysm involving visceral areas lies in how to reconstruct the five branches of the abdominal trunk artery, superior mesenteric artery, bilateral renal artery, and accessory renal artery, "Deng Hongping said. In order to safely and thoroughly cure Mr. Liu’s aortic dissection aneurysm, vascular surgery combined with anesthesia, nephrology, neurology, and critical care medicine for preoperative consultation and discussion, and finally decided to innovate on the top surgical procedure by performing" abdominal aortic aneurysm endovascular isolation surgery+bundle diameter five window surgery "for him. This procedure does not involve open chest or abdominal surgery, and the doctor only operates within the vascular lumen, allowing for minimally invasive repair of thoracic and abdominal aortic dissection aneurysms.
On July 10th, after careful preoperative preparation, the team successfully completed the surgery according to the preoperative plan. Mr. Liu successfully underwent extubation and resuscitation after the surgery, and was transferred back to the vascular surgery ward the next day without any obvious complications. Postoperative CTA re examination showed that Mr. Liu’s original dissection aneurysm was completely isolated, and all five branch arteries had good blood supply.
3D printing assisted extracorporeal stent window opening
It is reported that the "four window technique" is currently internationally recognized as the most suitable method for reconstructing large blood vessel branches. The bundle diameter technique is a top-level vascular surgical technique that sews the stent onto a 0.018-inch guide wire like a hinge. The stent is not fully unfolded, and after successful selection of all branch arteries, the bundle diameter guide wire is removed to completely release the main stent.
Deng Hongping stated that the "composite surgical technique" implemented for Mr. Liu is a combination of these two cutting-edge technologies, which has the advantage of facilitating the selection of branch arteries in the bundle diameter state and ensuring that internal organs are not ischemic. The success of the surgery relies on precise measurement of preoperative aortic data, meticulous surgical planning, and superb surgical techniques. Due to the involvement of multiple organs and the great difficulty of surgery, only a very small number of hospitals at home and abroad can carry out such procedures.
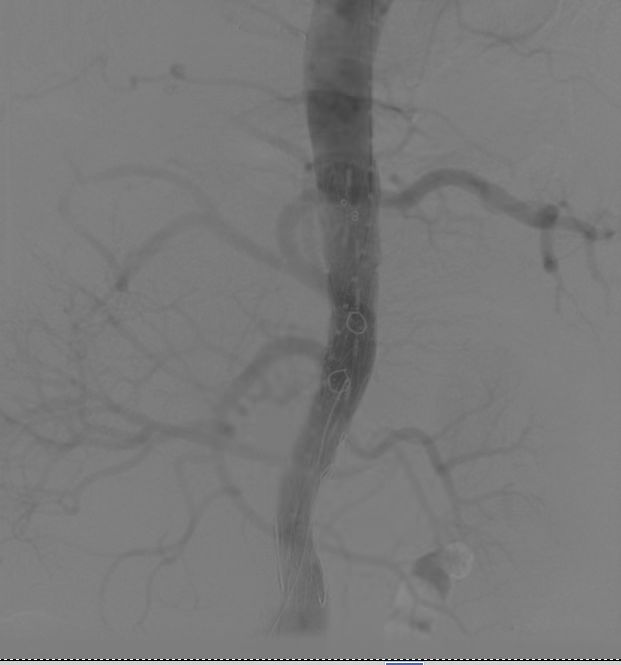
Aorta after angiography
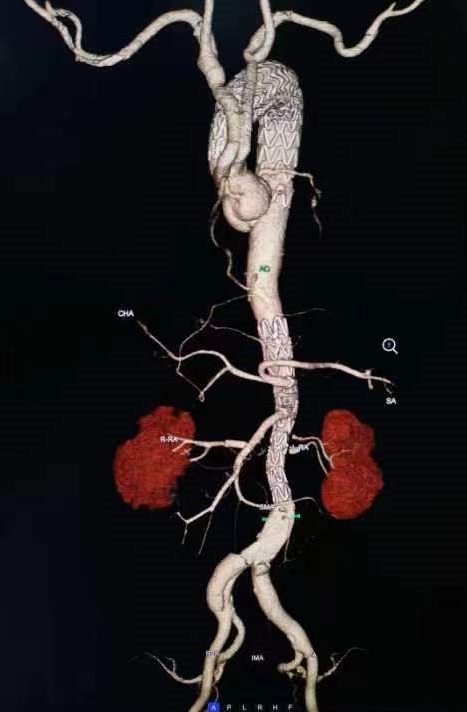
Postoperative CTA
Reminder:
High risk of large vessel diseases, early diagnosis and treatment are key
Deng Hongping introduced that aortic dissection is not a conventional tumor, it is like a bulging tire on a car; The aorta is the thickest blood supply artery in the human body, and if it swells and ruptures, the mortality rate is extremely high. Aortic dissection often tears from the thoracic aorta to the abdominal aorta, and even affects the iliac and femoral arteries, making it one of the most dangerous diseases.
After performing thoracic aortic surgery during the acute phase of dissection, although the risk of sudden death is greatly reduced, regular follow-up examinations are needed to determine whether the distal dissection has healed. A considerable number of patients may not heal or even gradually expand to form aneurysms. Abdominal aortic dissection often involves visceral arteries and has always been a difficult treatment point in vascular surgery.
For aortic dissection, the cutting-edge intracavitary minimally invasive surgery carried out by the Department of Vascular Surgery at Wuhan University People’s Hospital has developed rapidly, with advantages such as minimal trauma and fast recovery. However, it requires high technical requirements, especially for multi arterial branch reconstruction window surgery, which requires opening corresponding "windows" on the stent at the visceral artery position of the main stent. Individualized design and super selection of branch stents are necessary to ensure that the blood supply organs are not ischemic and completely isolated without leakage. The difficulty of accurately opening and selecting windows is extremely high. Once one window is right or wrong, all windows may be misaligned, leading to ischemic necrosis of the branch artery blood supply organs, and the surgery is extremely delicate and complex.
Encountering another aneurysm after aortic dissection surgery! Wuhan University People’s Hospital innovates minimally invasive ’bomb disposal’ for patients
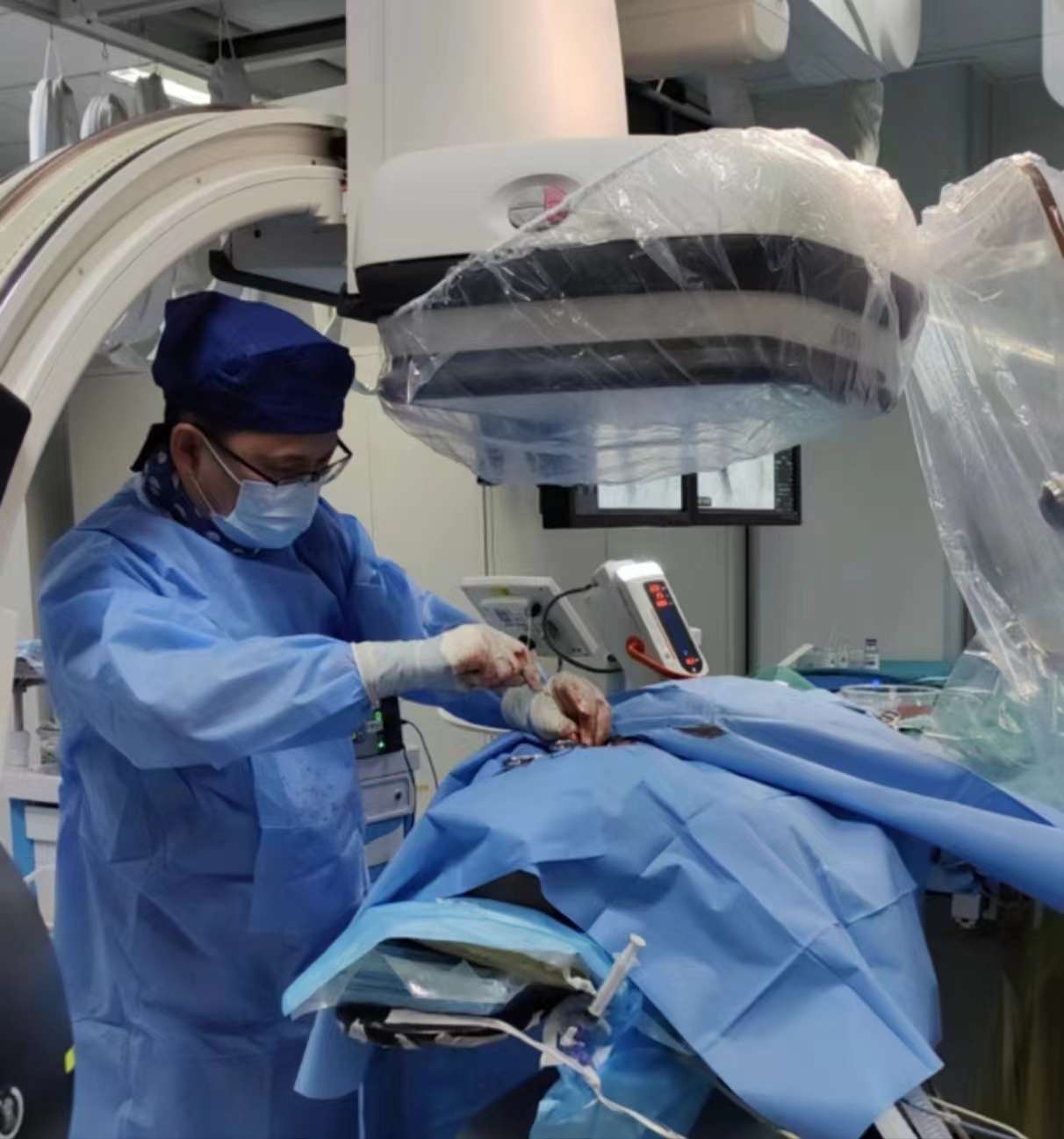
Super selected abdominal aortic branch vessels
The successful implementation of such surgical procedures marks that the surgical treatment of aortic diseases in the Department of Vascular Surgery at Wuhan University People’s Hospital has entered the era of "fully minimally invasive" surgery, which can benefit more patients.
Deng Hongping reminds that if hypertensive patients find pulsatile masses, or experience chest, back, or abdominal tearing, electric shock, or cutting pain, they should be highly alert to the occurrence of aortic aneurysm or aortic dissection and seek treatment at a regular hospital as soon as possible. After aortic surgery, it is necessary to control blood pressure and heart rate, treat chronic cough and constipation, correct unhealthy habits such as smoking, drinking, and staying up late, and follow up regularly with doctors according to their advice.
This article is reprinted from "Dingxiangyuan"
Surgerycast
Shanghai Headquarter
Address: Room 201, 2121 Hongmei South Road, Minhang District, Shanghai
Tel: 400-888-5088
Email: surgerycast@qtct.com.cn
Beijing Office
Address: room 709, No.8, Qihang international phase III, No.16, Chenguang East Road, Fangshan District, Beijing
Tel: 13331082638( Liu )
Guangzhou Office
Address: No. 15, Longrui street, longguicheng, Taihe Town, Baiyun District, Guangzhou
Tel: 13302302667 ( Ding )