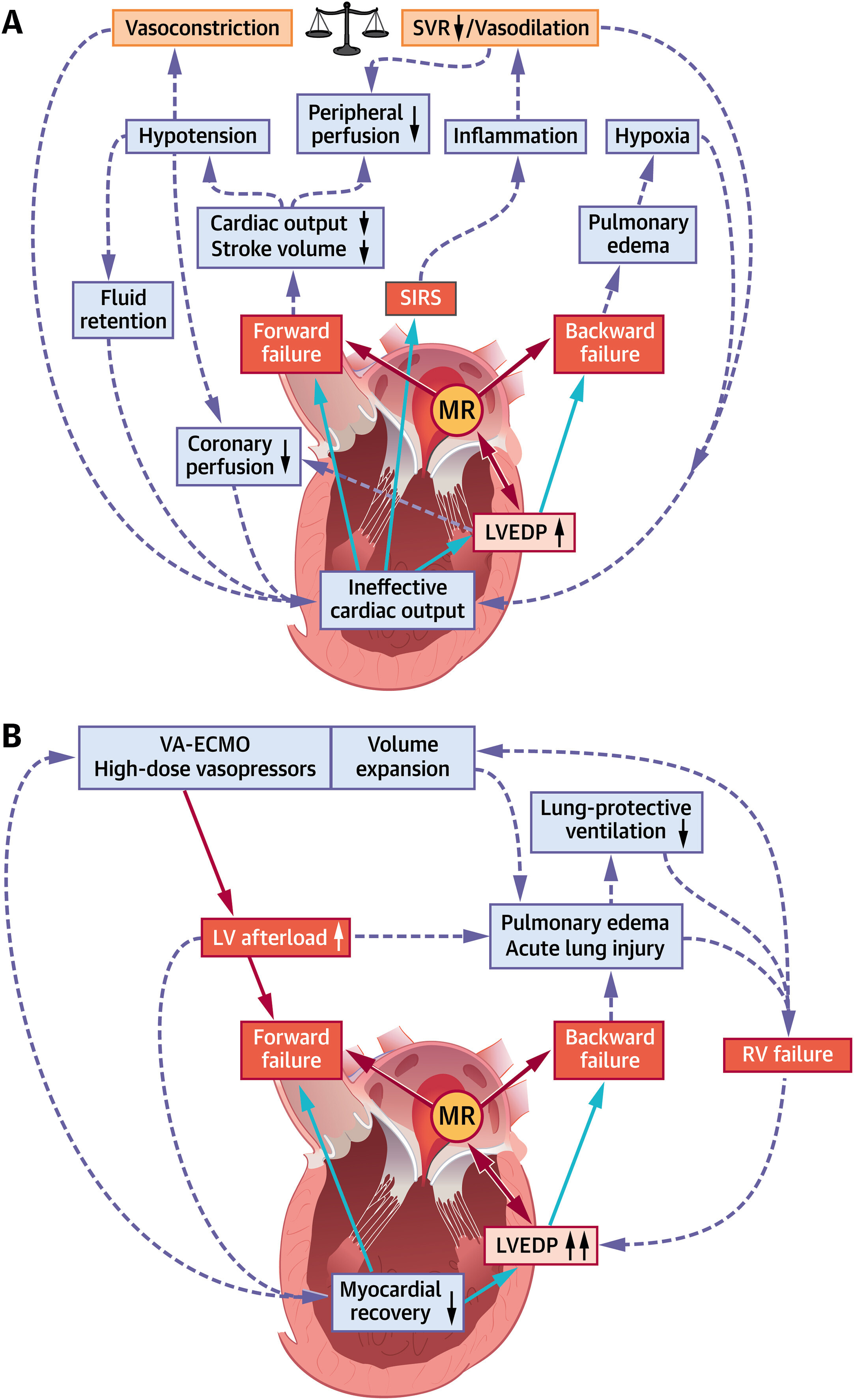
In recent years, transcatheter mitral valve edge-to-edge repair (M-TEER) has been established as an evidence-based therapy in patients with mitral regurgitation (MR)–dominant phenotypes of chronic heart failure with reduced ejection fraction (HFrEF) on optimized medical therapy. In striking contrast to the dynamic evolution of both medical and device-based therapeutic options in chronic HFrEF, care for patients with the most acute form of heart failure, ie, cardiogenic shock (CS), has seen considerably less advancements over time and remains one of the most challenging situations in cardiac intensive care. Apart from reversal of the underlying cause of CS, care for the majority of patients with CS is rather supportive by addressing the ineffective cardiac output as well as its deleterious multisystemic downstream effects (Figure 1A).
MR and Pathophysiological Cascades of Cardiogenic Shock
(A) Increased left ventricular end-diastolic pressure (LVEDP), forward and backward failure in cardiogenic shock trigger acute and subacute derangements to the circulatory system. All 3 parameters are mitral regurgitation (MR)–dependent. (B) Mechanisms sustaining left ventricular (LV) afterload increase and unloading in advanced cardiogenic shock management. RV = right ventricular; SIRS = systemic inflammatory response syndrome; SVR = systemic vascular resistance; VA-ECMO = venoarterial extracorporeal membrane oxygenation.
About 10% of patients with acute myocardial infarction–associated CS display severe MR. These patients share the devastating prognosis of CS with in-hospital mortality rates up to 60%, and mortality remains as high as 40% even in those patients who are successfully bridged to mitral valve surgery. Interestingly, more recently published data from a patient-level meta-analysis of small-scale cohorts suggest that M-TEER may markedly improve short- and intermediate-term outcome in patients with CS and MR.
In this issue of the Journal of the American College of Cardiology, Simard et al report on an analysis of 3,797 patients with CS in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry who underwent M-TEER for moderate or severe mitral regurgitation. There are 2 main findings of this study. First, the data by Simard et al suggest that device success during M-TEER in patients with CS and significant MR (defined as final MR grade ≤2+ and MR reduction ≥1 absolute grade) is associated with improved survival in-hospital and at 30 days. This finding is in line with previous analyses, but founded on a substantially larger number of patients. Second, among 2,773 patients eligible for follow-up, successful MR reduction was associated with lower all-cause mortality and heart failure hospitalizations at 1 year. Notably, the association between device success and improved outcomes was consistent in several clinically important subgroup analyses, including patients with different CS inclusion criteria, MR etiology, and more profound impairment in left ventricular (LV) systolic function.
The work by Simard et al is the largest and most robust analysis on the benefit of M-TEER in patients with CS and MR so far. It raises hope that M-TEER may expand the limited therapeutic options to improve the often dramatic clinical course and poor outcome of patients in CS. Yet, as correctly pointed out by the authors, there are caveats in the interpretation of their data, and most of them are inherent to the nature of a retrospective registry-based analysis. One relevant issue relates to the definition of CS in the Society of Thoracic Surgeons/American College of Cardiology TVT (Transcatheter Valve Therapy) Registry, which solely relies on the registry variables cardiogenic shock (defined as persistent hypotension along with a reduction in cardiac index), inotrope use, or mechanical circulatory support. Hence, although there is certainly a low-output situation in the present cohort, it remains difficult to judge on the severity of CS because information on clinical and biochemical manifestations of inadequate tissue perfusion, a pathophysiological hallmark of CS, is lacking. In addition, the etiology of CS is difficult to infer from the available registry data, especially in conjunction with the strikingly high percentage of patients with degenerative MR in this CS cohort. Even considering a bias introduced by the later approval of M-TEER for functional MR in the United States, an acute clinical presentation with cardiac hemodynamic instability requiring inotropes or mechanical circulatory is rather unusual in patients with chronic degenerative MR. Acute degenerative MR as a mechanical complication of myocardial infarction seems more plausible in this setting, but such a valvular etiology of CS has become rare nowadays. Furthermore, coronary artery disease was excluded in more than 40% of patients, suggesting that inciting events other than ischemia-driven LV dysfunction were involved in the development of CS. Such insights not only point toward certain heterogeneity within the present patient cohort, but also underline the importance of including a more detailed phenotyping into the planning process of future studies in this area. Taken together, these and other limitations (indication bias, immortal bias, lack of core laboratory adjudication of MR severity) preclude definitive conclusions regarding the efficacy of M-TEER in this cohort. Notwithstanding such criticism, the real-world data provided in this study are important and clearly pave the way for carefully designed clinical trials to test whether the encouraging prognostic implications of M-TEER in CS are validated in a randomized controlled setting. The CAPITAL MINOS (Transcatheter Mitral Valve Repair for Inotrope Dependent Cardiogenic Shock) multicenter, open-label, randomized controlled trial is likely the first study addressing the question whether M-TEER is superior to standard of care medical therapy alone in improving the in-hospital outcome of patients with CS and significant MR. The trial recruits patients with stage C or D cardiogenic shock according to the Society for Cardiovascular Angiography and Interventions classification system who require persistent inotrope, vasopressor, or nondurable mechanical circulatory support, or in whom weaning of ventilatory support fails because of pulmonary edema.
Provided that upcoming trials confirm currently available data, M-TEER has the potential to significantly change clinical care for patients with CS, but will also shed new light on the role of MR in the pathophysiology of CS. A significant rise in LV end-diastolic pressure is at the center of hemodynamic perturbations in CS and results in backward failure as well as further impairment of myocardial contractility (Figure 1A). A complex vicious circle is initiated, in which forward failure, backward failure, alterations of peripheral vascular resistance, and systemic inflammatory responses negatively influence each other and cause rapid clinical deterioration with multiorgan dysfunction. From a hemodynamic point of view, MR in the acute failing heart is rather a double-edged sword. On the one hand, MR sustains LV volume overload and may, therefore, aggravate the adverse effects of a LV end-diastolic pressure increase, particularly in the early stages of CS. In this respect, M-TEER would be an attractive therapeutic option to reduce LV preload and thereby LV end-diastolic volume, as observed after treatment of functional MR in patients with stable HFrEF. On the other hand, MR may mediate a “pop off” effect for the acute failing LV in CS, and the abrupt increase in afterload after M-TEER bears the risk of further left ventricular impairment. Lessons learned from M-TEER in stable HFrEF suggest that a decrease in LV preload caused by MR reduction might attenuate unfavorable fiber stretch and thereby compensate for the rise in LV afterload. Yet, if this holds true for CS remains unknown. Finally, an even more complex scenario is encountered in CS patients with increased peripheral vascular resistance on high-dose vasopressors and, even more important, in CS patients on venoarterial extracorporeal membrane oxygenation. These patients may face a continuous adverse increase in LV afterload (Figure 1B) with further attenuation of LV forward flow, limiting unloading of the LV. A benefit of M-TEER in this situation is highly questionable.
With its application in CS, M-TEER begins another journey in a highly complex clinical scenario. Wisely chosen designs of clinical trials, a thorough characterization of patients included in trials, and careful subgroup analyses will be crucial to ultimately address the question if (and for whom) there is a benefit of M-TEER in CS. It seems unlikely that every patient with CS will derive benefit. Yet, it is worth the effort keeping in mind the fate of our CS patients.
This article is reproduced from JACC journals.
Surgerycast
Shanghai Headquarter
Address: Room 201, 2121 Hongmei South Road, Minhang District, Shanghai
Tel: 400-888-5088
Email: surgerycast@qtct.com.cn
Beijing Office
Address: room 709, No.8, Qihang international phase III, No.16, Chenguang East Road, Fangshan District, Beijing
Tel: 13331082638(Liu Jie)
Guangzhou Office
Address: No. 15, Longrui street, longguicheng, Taihe Town, Baiyun District, Guangzhou
Tel: 13302302667